What is an Atom? Definitions & Examples Let us learn Basics News Bugz
Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Since the iodine is added as a 1− anion, the number of electrons is 54 [53 - (1-) = 54]. Exercise 2.2.1 2.2. 1. An ion of platinum has a mass number of 195 and contains 74 electrons.
Atomic Structure (GCSE) — the science hive
All atoms are roughly the same size, whether they have 3 or 90 electrons. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch). A convenient unit of length for measuring atomic sizes is the angstrom (Å), defined as 10 −10 metre. The radius of an atom measures 1-2 Å.
Atomic Structure Broad Learnings

The Structure of an Atom: Parts, Diagram, Examples Anything that has mass and occupies space is called matter. The matter is made up of atoms. Atomic structure is the structure of an atom that consists of a nucleus (the centre), protons (positively charged), and neutrons (neutral).
The Nucleus of the Atom and Radioactivity

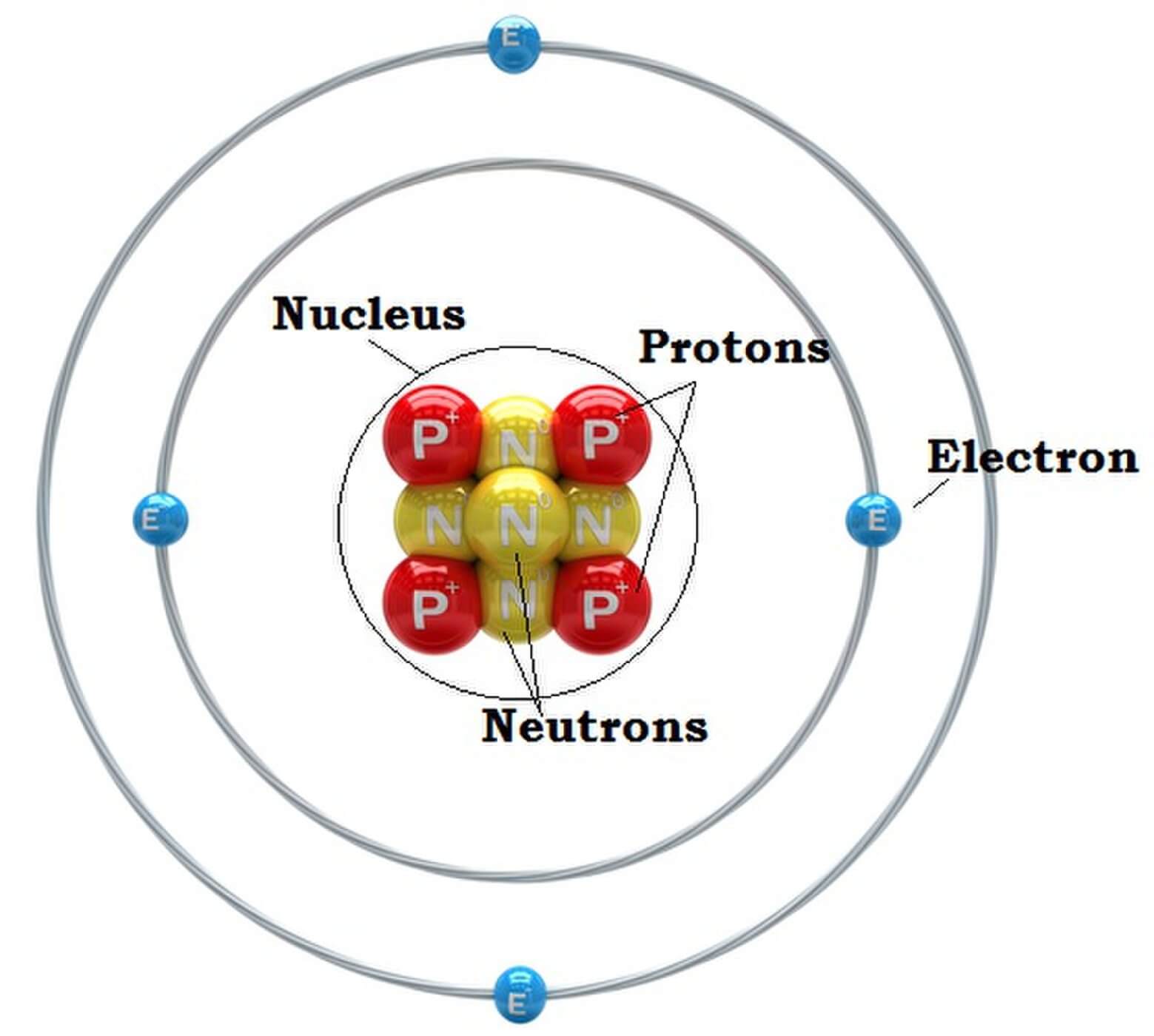
1. Draw five protons in the nucleus of the atom. Label them with their charge. 2. Draw six neutrons in the nucleus of the atom. 3. Draw two electrons in the first energy level and label them with their charge. 4. Draw three electrons in the second energy level and label them with their charge. 5. What element is represented by the diagram?
35 Label The Parts Of The Atom In The Diagram Below Labels For Your Ideas
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
Atom Diagram [/caption]The image on the left is a basic atom diagram. This one shows the protons, neutrons, and electrons of a carbon atom. Each is in a group of six. That makes the atom.
Lets Get Inside An Atom!! The Science Station
Parts of the Atom Part 1 - Label the parts of the atom below (protons, neutrons, electrons, nucleus, quarks). + + +----+ to the nucleus)? Part 2 - Answer the following questions. 1. _____What part of the atom has no charge? 2. _____What part of the atom has a positive charge? 3. _____What part of the atom has a negative charge? 4.
The Structure Of An Atom Explained With A Labeled Diagram Best Diagram Collection
Key Points. An atom is composed of two regions: the nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Protons and neutrons have approximately the same mass, about 1.67 × 10 −24 grams, which scientists define as one atomic.
Atoms, Molecules, and Compounds What's the Difference? Owlcation
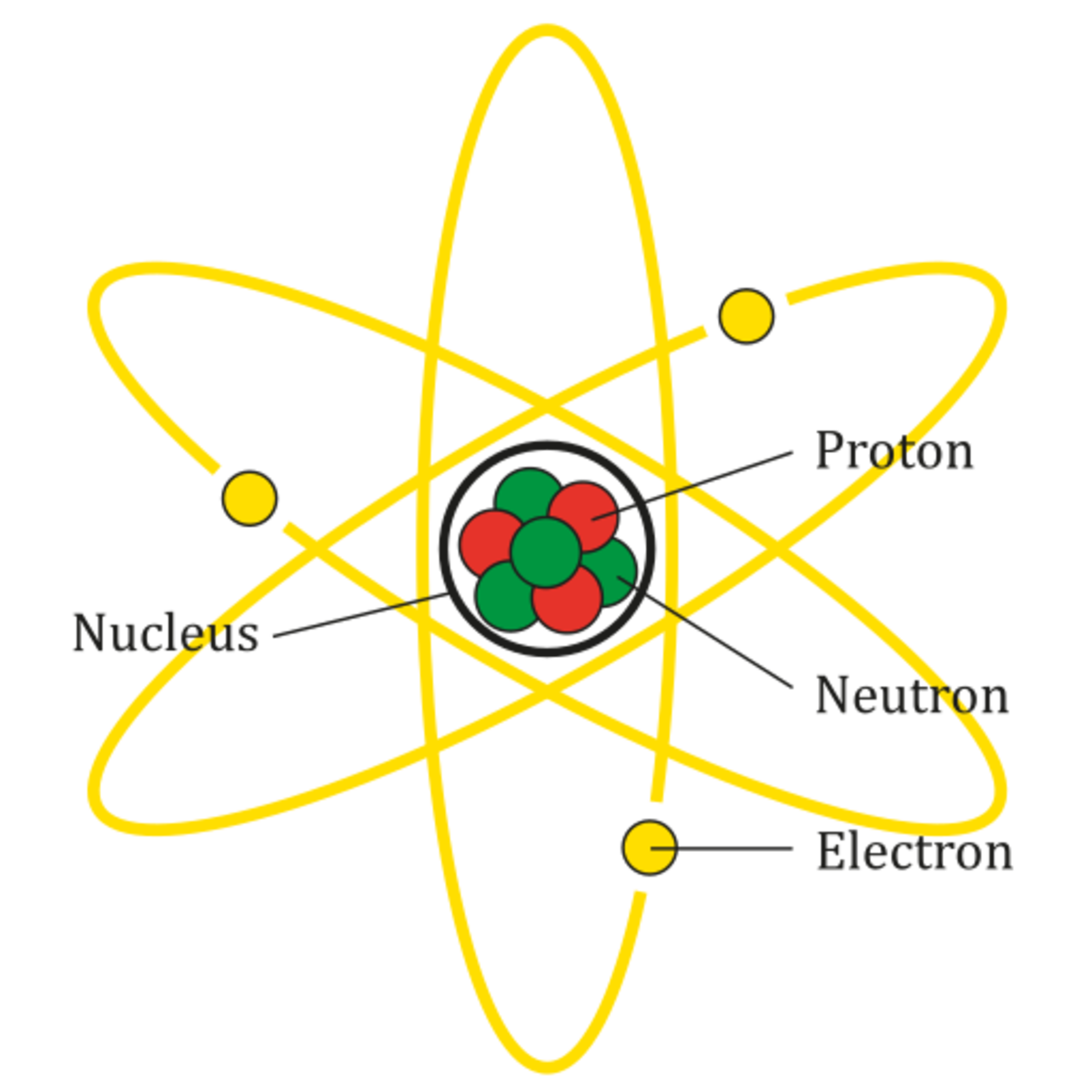
Figure 2.2.1 2.2. 1: The Structure of the Atom. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different.
Learn the Parts of an Atom

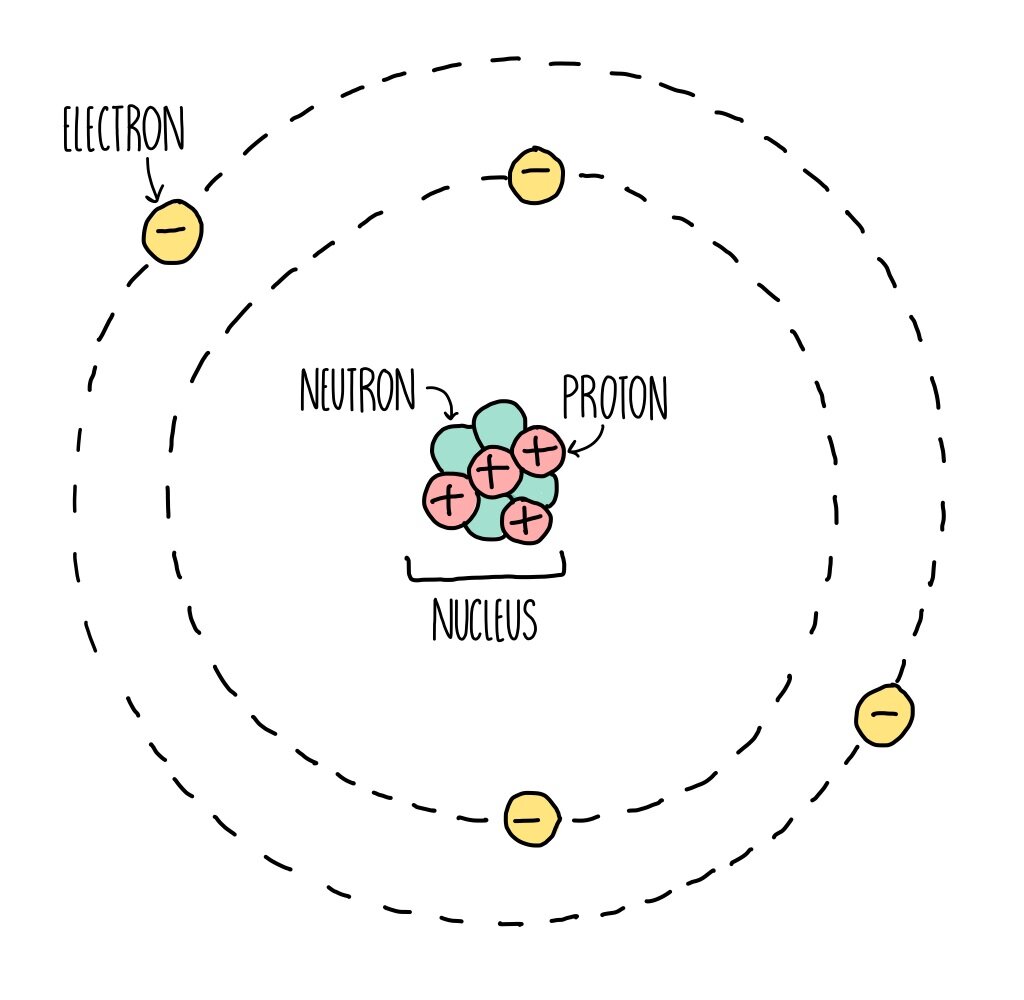
Basic Diagram of an Atom Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The center of an atom is the nucleus and one or more electrons surrounding the nucleus.
Structure of an Atom Structure & Use of Electron & Proton in Electronics
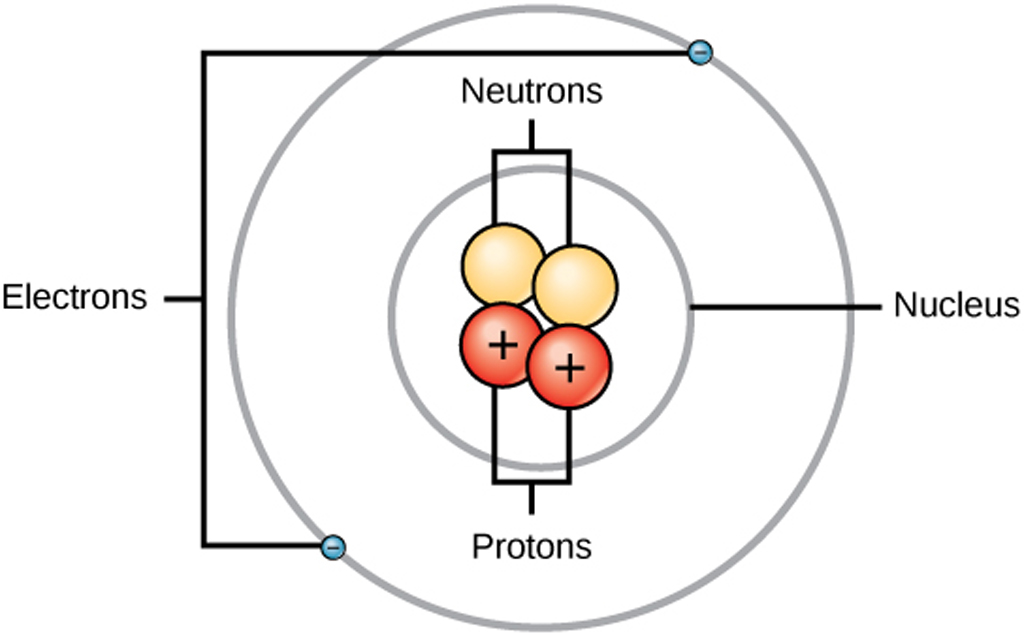
In this video we cover the structure of atoms, what are subatomic particles, energy levels, and stable and reactive atoms.Transcript and notesAtomic structur.
What is Atom? How does it Exist? and it's Symbols Teachoo
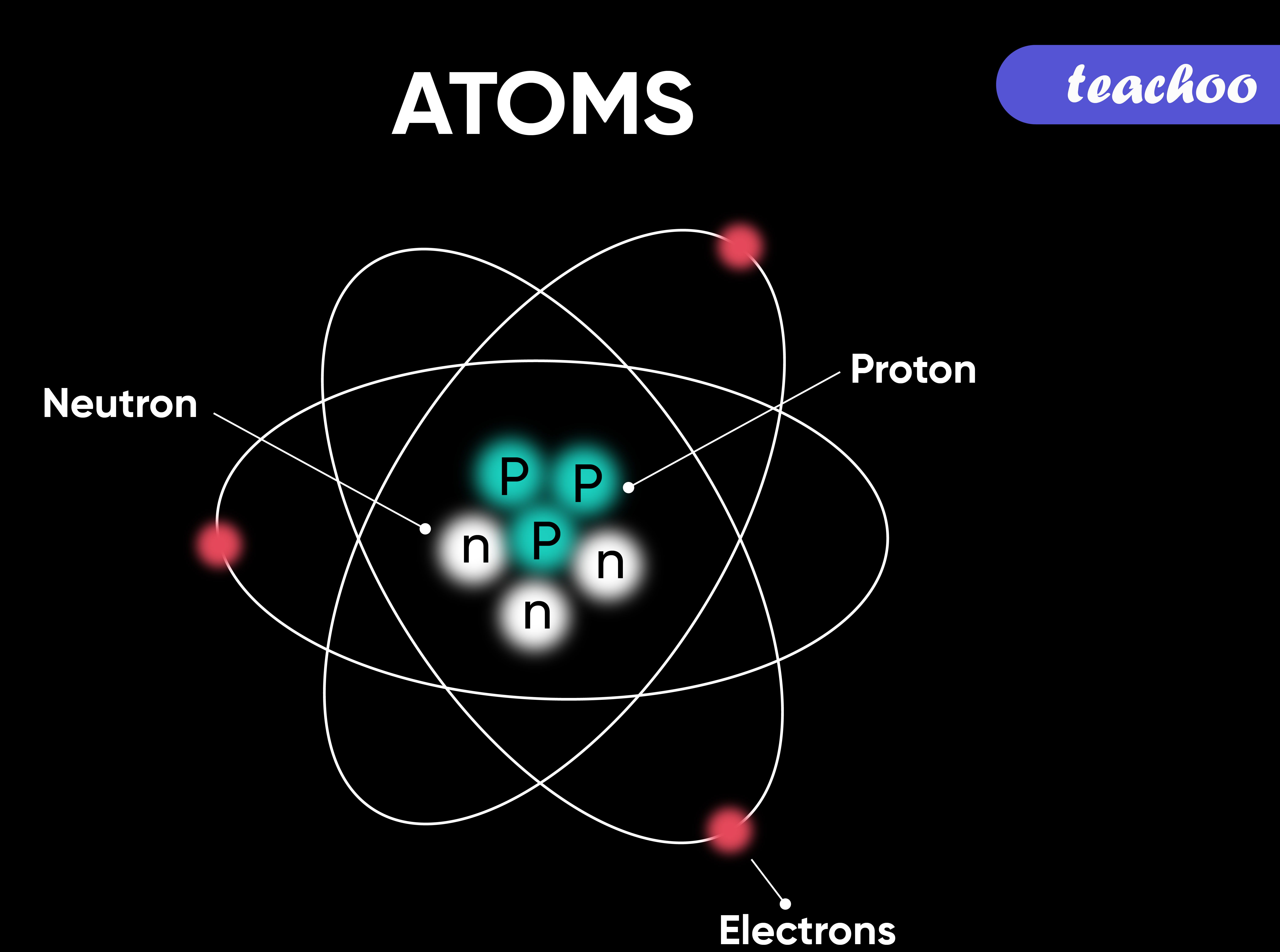
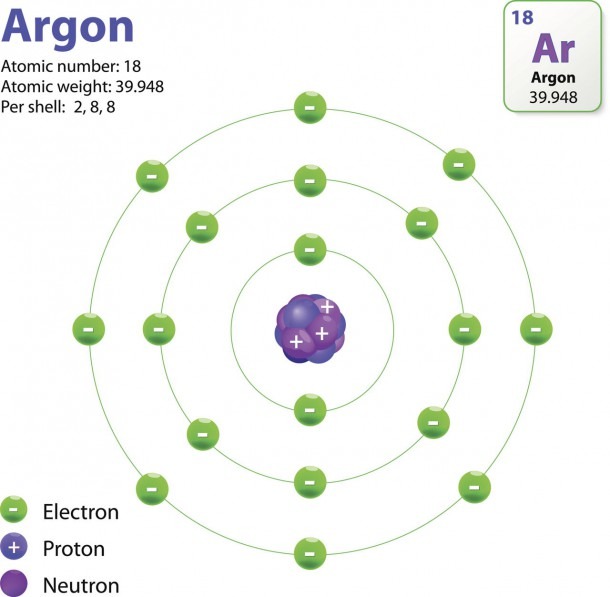
The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun.
Label Parts of an Atom — Learning in Hand with Tony Vincent
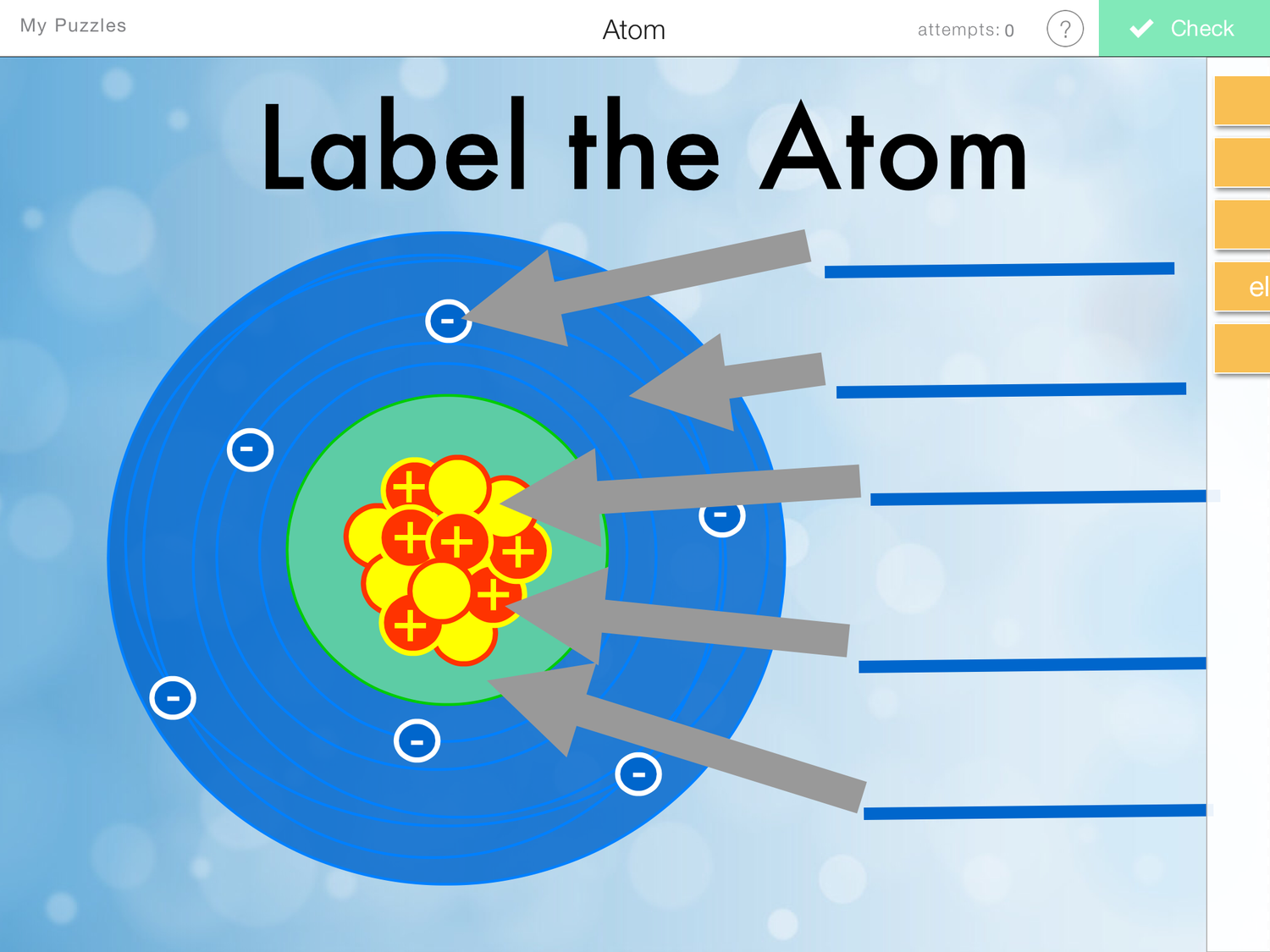
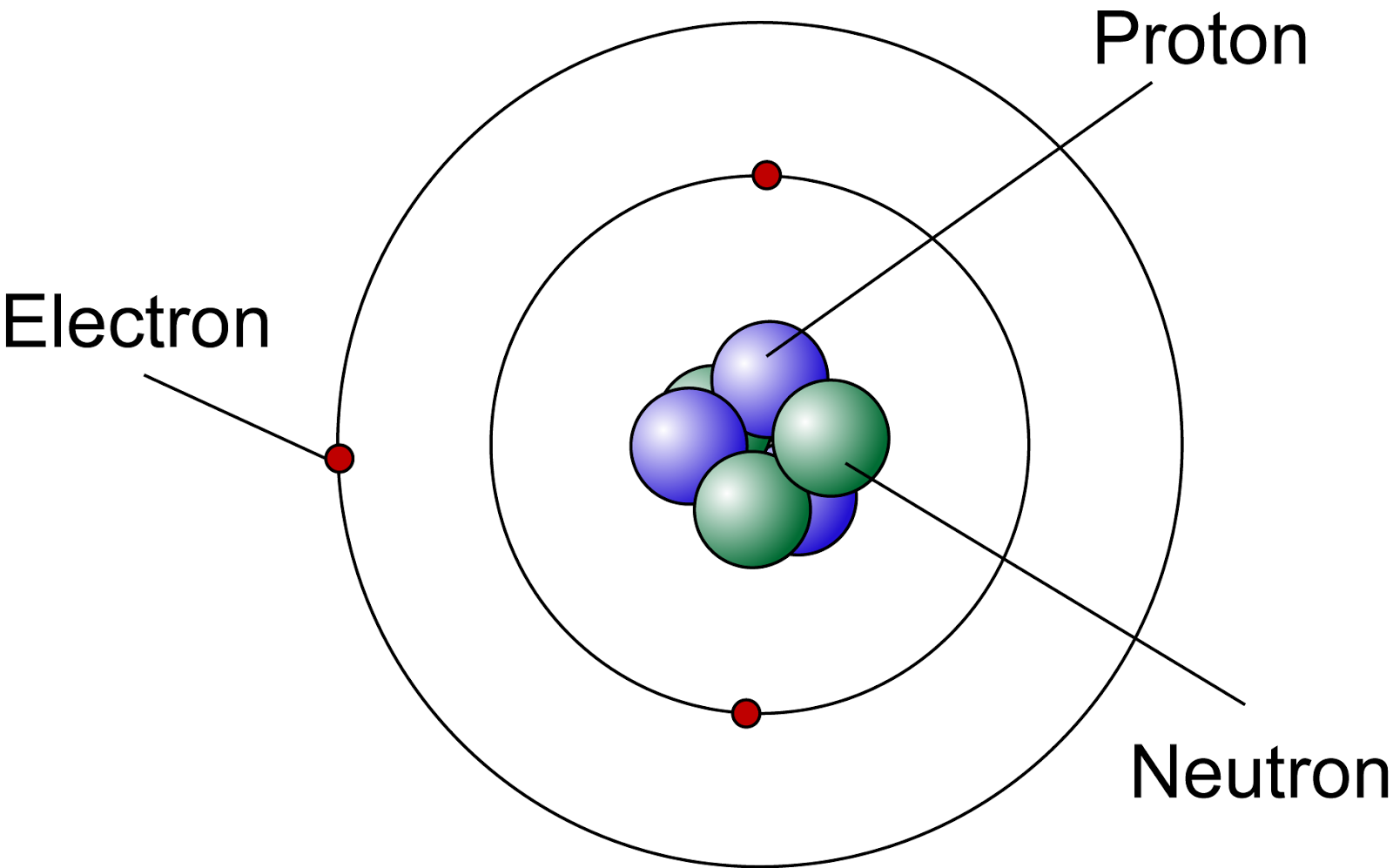
Figure three is a schematic diagram of a helium atom in its lowest energy state. Two protons are present in the nucleus of all helium atoms. In the most common variety of helium, the nucleus also contains two neutrons, which have nearly the same mass as the proton but carry no charge. Two electrons orbit the nucleus.
Atoms & Molecules echapter — The Biology Primer
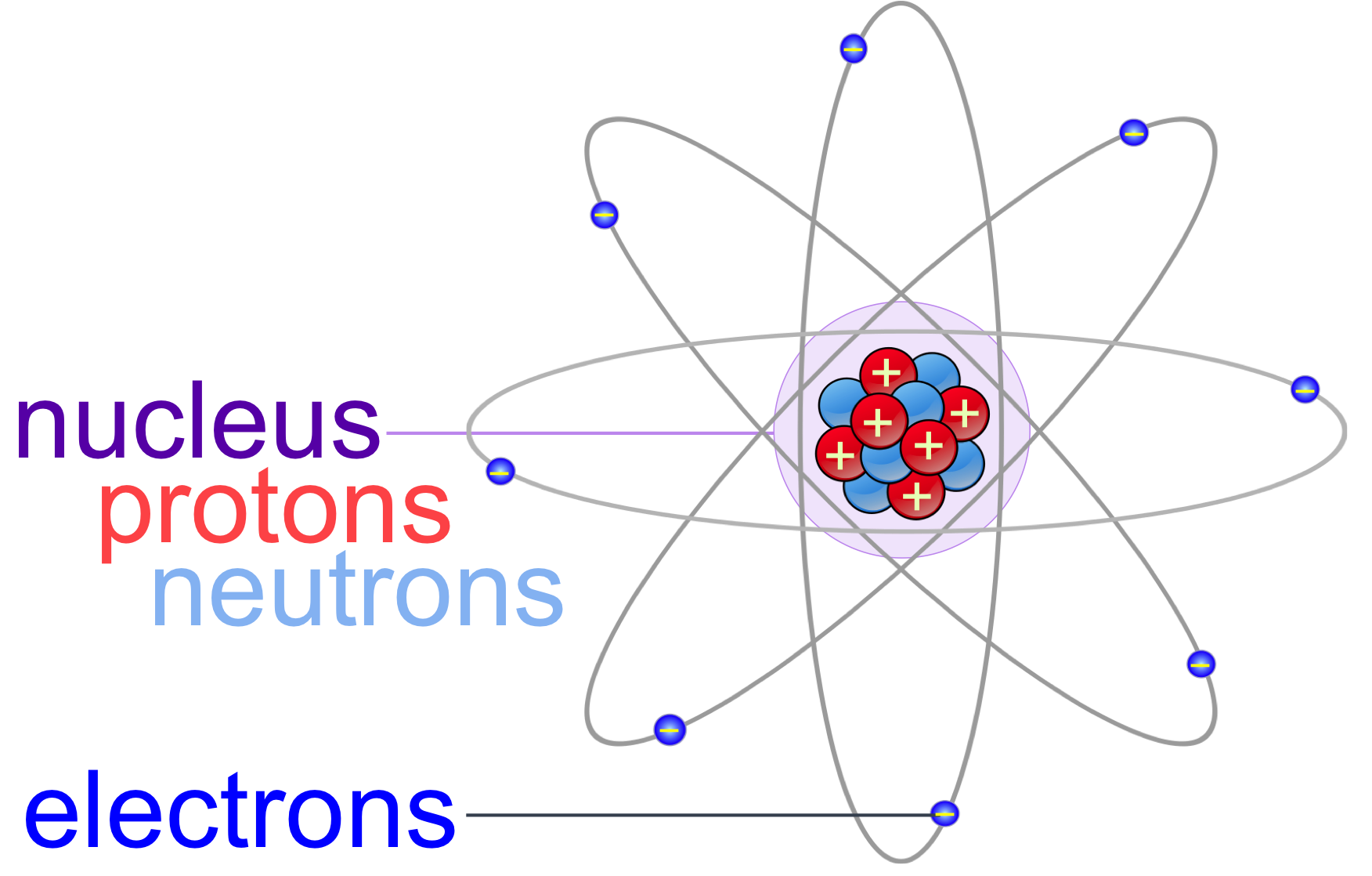
Physical Chemistry (Essentials) - Class 11 8 units · 52 skills. Unit 1 Welcome to physical chemistry. Unit 2 Structure of atom. Unit 3 Some basic Concepts of Chemistry. Unit 4 Redox reactions. Unit 5 Gaseous state. Unit 6 Thermodynamics. Unit 7 Chemical Equilibrium. Unit 8 Ionic equilibrium.
Atomic structure teaching resources the science teacher
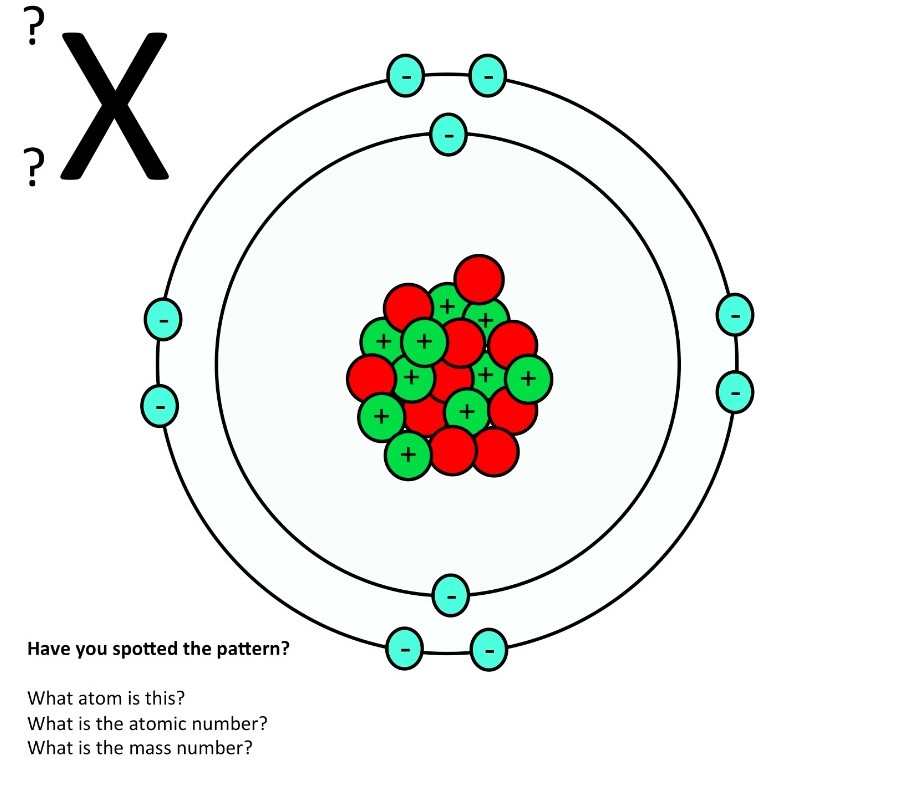
Atom The atom is the basic particle of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically-bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms.
Skills Practice AMAZING 8TH GRADE SCIENTISTS
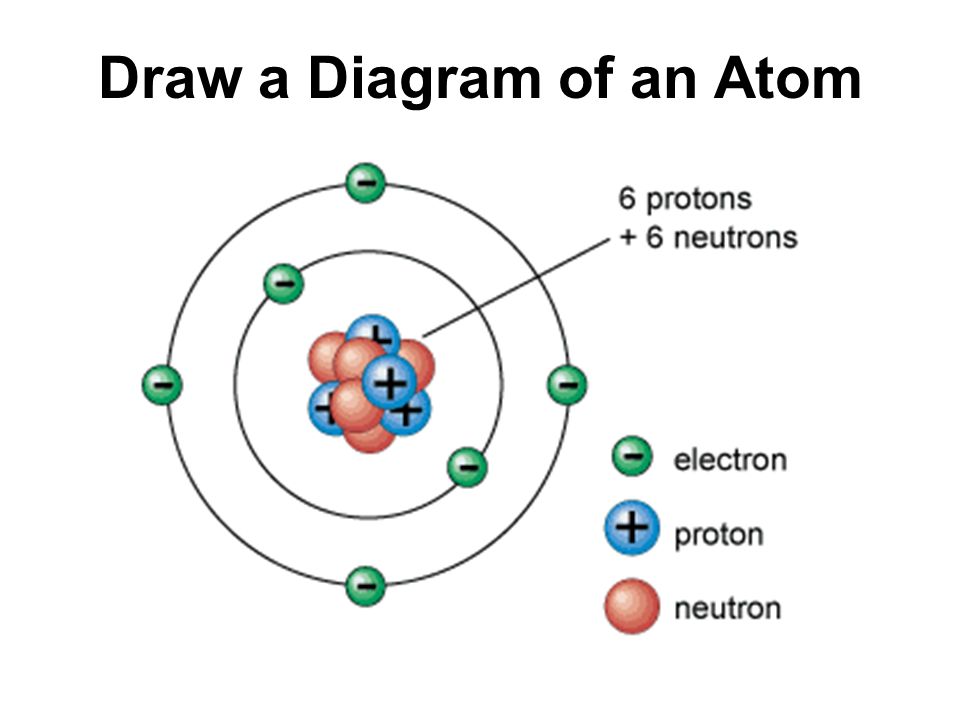
proton. Definition. Positively charged particle found in the nucleus of an atom. Location. Term. nucleus. Definition. The center of the atom which contains protons and neutrons. Location.
atom diagram to label
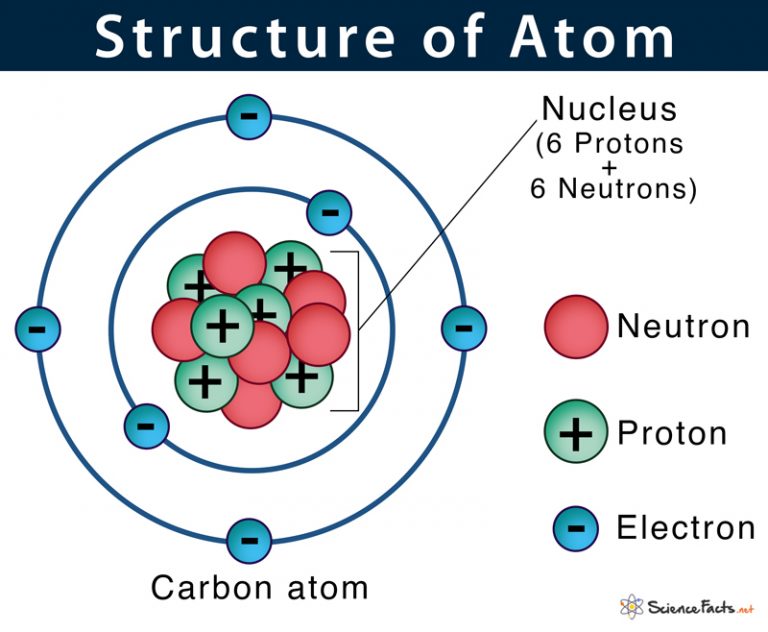
An ion of an atom is one in which the number of protons and electrons is not the same. If there are more protons than electrons, an atomic ion has a positive charge and is called a cation. If there are more electrons than protons, the ion has a negative charge and is called an anion.