PPT Bonding General Concept PowerPoint Presentation, free download

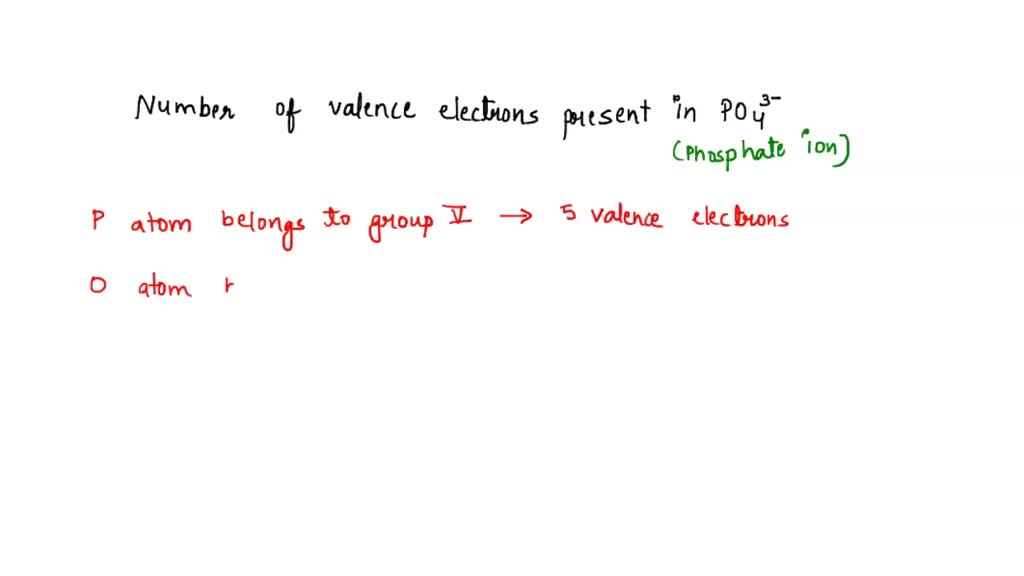
Drawing the Lewis Structure for PO 4 3-Viewing Notes: PO 4 3-has a charge of -3 (that makes it a negative ion or anion). That means that it has an three extra electrons that needs to be taken into account.. Let's do the Lewis structure for PO4 3-. Phosphorus has 5 valence electrons. Oxygen has 6, we've got 4 Oxygens. This negative 3 up here.
SOLVED The formal charge on the phosphorua atom in resonance structure
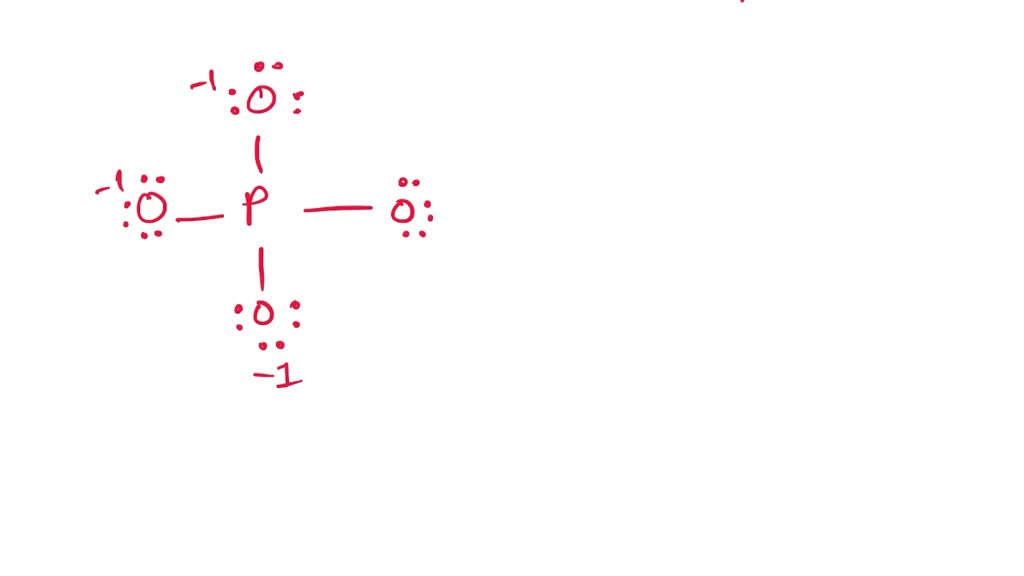
PROBLEM 4.2.4 4.2. 4. Methanol, H 3 COH, is used as the fuel in some race cars. Ethanol, C 2 H 5 OH, is used extensively as motor fuel in Brazil. Both methanol and ethanol produce CO 2 and H 2 O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas.
Structure of PO43 ion Phosphate ion structure Lewis dot method

Phosphate is a very weak oxidizing agent. Since the phosphorus is in its highest oxidation state in phosphate ion, this ion cannot act as a reducing agent. This page titled Phosphate Ion (PO₄³⁻) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by James P. Birk. Phosphate ion is a reasonably strong base.
Resonance Structures for PO4 3 (Phosphate ion) YouTube

A step-by-step explanation of how to draw the PO43- Lewis Dot Structure (Phosphate ion).For the PO4 3- structure use the periodic table to find the total num.
PO4 3 Lewis Structure How to Draw the Dot Structure II lSCIENCE ll
In this video, Let us discuss how to write Lewis structure of PO43-, Phosphate ion easily. A simple notation used to represent valence electrons in an atom is called Lewis symbol. According.
Lewis Structure, Hybridization, Polarization, and Molecular Geometry of

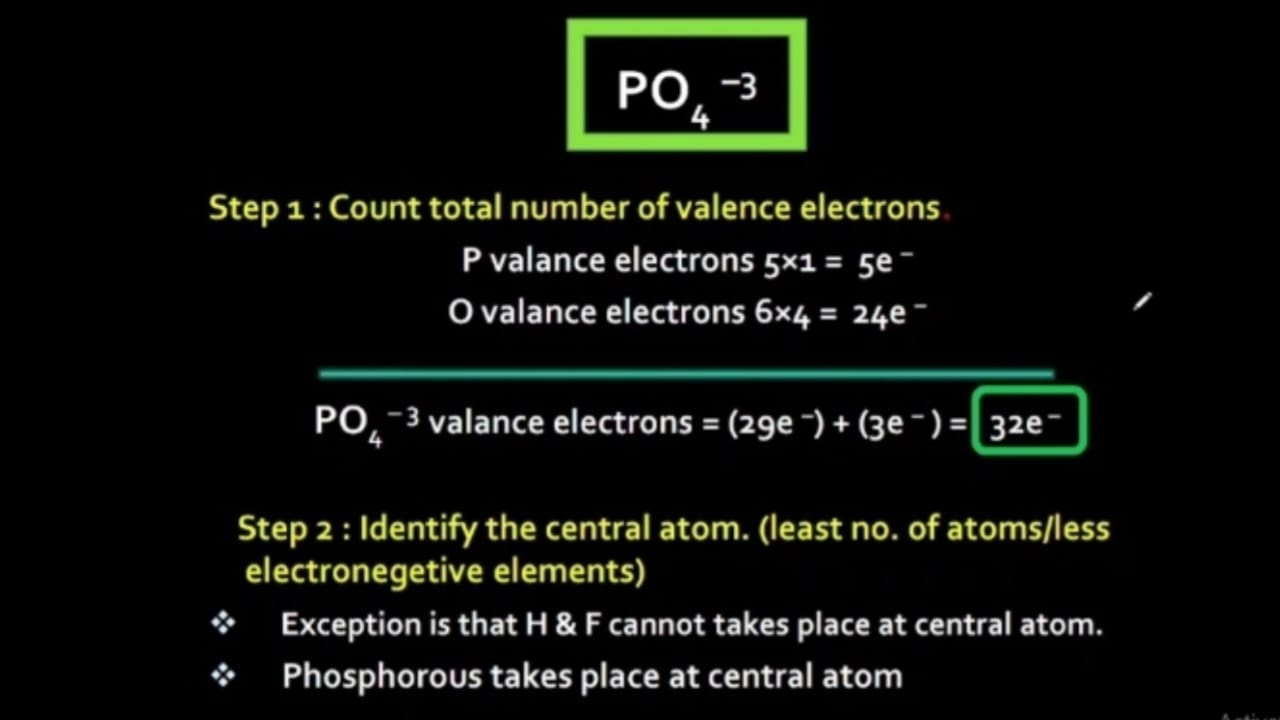
Steps of drawing PO4 3- lewis structure Step 1: Find the total valence electrons in PO4 3- ion. In order to find the total valence electrons in PO4 3- ion (phosphate ion), first of all you should know the valence electrons present in phosphorus atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
PO43 lewis structure, molecular geometry, hybridization, and bond angle

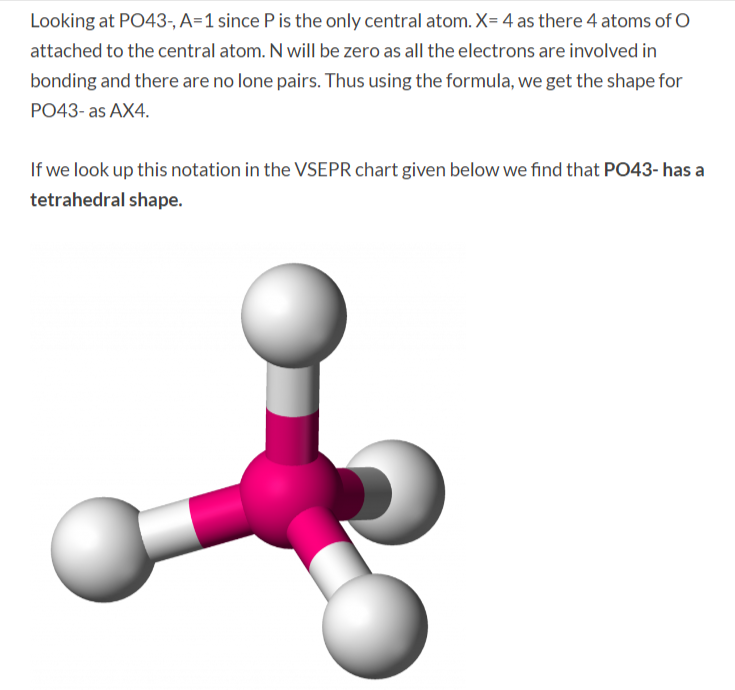
The PO4 3- ion has a Lewis structure with a central phosphorus atom bonded to four oxygen atoms. The phosphorus atom has a formal charge of +3, while each oxygen atom has a formal charge of -1. The Lewis structure of PO4 3- shows that it has a tetrahedral molecular geometry.
PO43LewisStructureHybridizationPolarityandMolecularGeometry
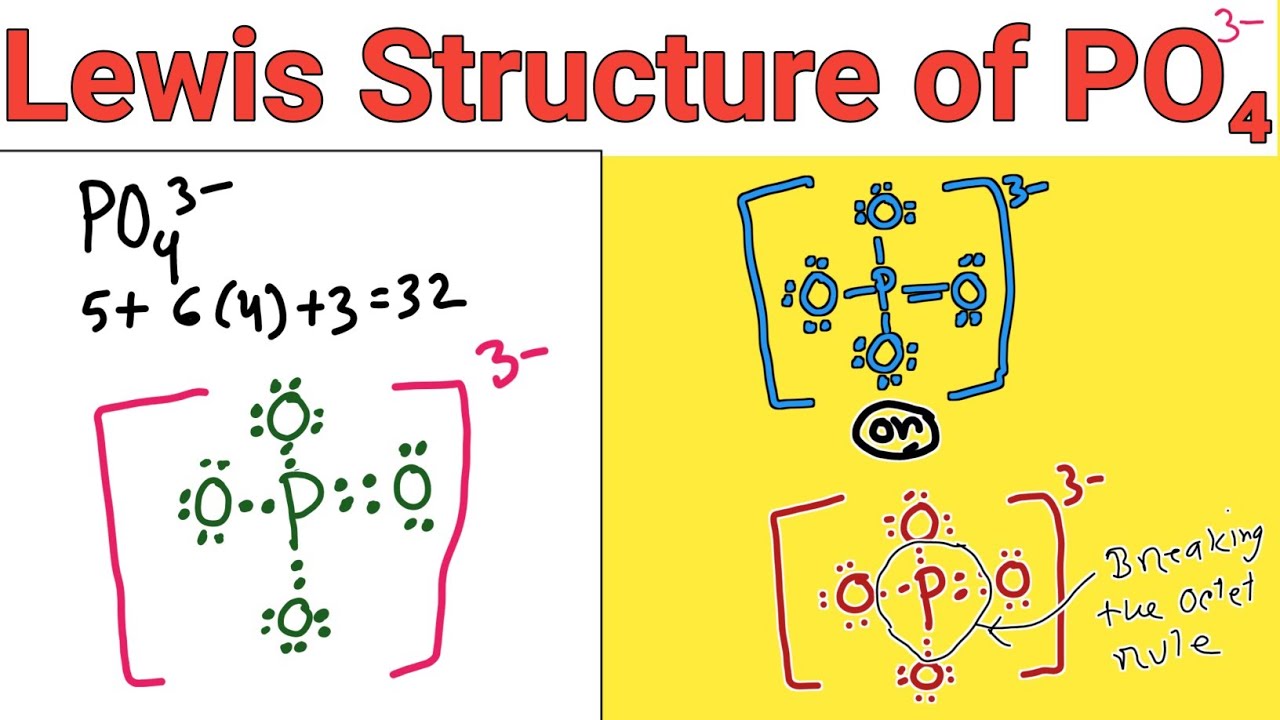
Geometry. PO43- Geometry and Hybridization. Phosphorous is the central and there are 5 + 4×6 + 3 = 32 electrons. Give each oxygen three lone pairs: All the electrons are used and the only thing to fix is the ionic charge. As drawn, it would be a -4, therefore, we give one lone pair from an oxygen to make a double bond with the P which can.
Simple Method for writing Lewis Structures of the phosphate ion(PO4)3

In the Lewis structure of PO43- there are a total of 32 valence electrons. For the Lewis structure for PO4 3- you should take formal charges into account to find the best Lewis structure for the molecule. Remember, PO4 3- has a negative three charge on the molecule. For the Lewis structure you'll need to have a total charge for the molecule of 3-.
PO43 Molecular Geometry / Shape and Bond Angles YouTube

Lewis structure of PO43- ion (phosphate ion) contains one double bond and three single bonds between the Phosphorus (P) atom and Oxygen (O) atoms. The Phosphorus atom (P) is at the center and it is surrounded by 4 Oxygen atoms (O). Let's draw and understand this lewis dot structure step by step.
In PO43 , the formal charge on each oxygen atom and the P O bond order

In the PO 43- Lewis structure, there is one double bond and three single bonds around the phosphorus atom, with four oxygen atoms attached to it. One oxygen atom with a double bond has two lone pairs, and three oxygen atoms with single bonds have three lone pairs. Also, there is a negative (-1) charge on the three oxygen atoms with single bonds.
Resonance The resonance structure of the phosphate ion (PO4(3)) YouTube
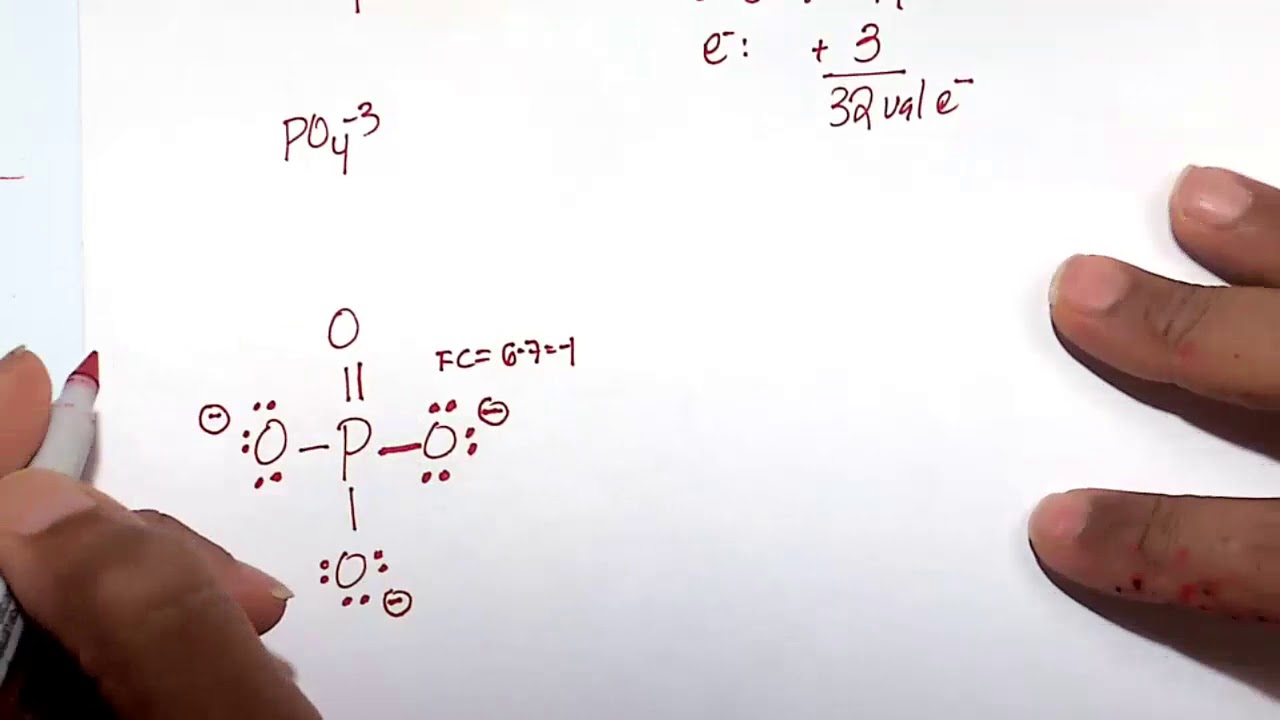
The Lewis structure for PO43- is shown.
Lewis Dot Structure of Phosphate (PO4 3).....No More Confusion

Hey Guys!Did you know that Phosphorus can have expanded orbitals and can accommodate more than 8 electrons in its outer shell? Well, such information helps t.
SOLVED 23) Examine this Lewis structure for the phosphate ion, PO43
This chemistry video tutorial explains how to draw the lewis structure of PO4 3-, the phosphate ion. It also discusses the formal charge and resonance struc.
Lewis structure of PO4 3 (Phosphate ion) YouTube
Lewis structure of phosphate ion is drawn clearly in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of PO 43- ion. In lewis structure, there should be charges on atoms. Phosphate ion | PO 43- Phosphate ion is one of the oxyanion of phosphorous. Phosphorous is at +5 oxidation state in PO 43-.
PO43 Lewis Structure (Phosphate Ion) YouTube

Lewis structure of PO 43- ion is important because it is required to draw resonance structures of phosphate ion. Resonance structures of PO 43- ion Let's draw four stable four resonance structures for the phosphate anion (NO 3- ). Lone pairs, charges and bonds of PO 43- ion